
we think about measurement
Hydrogen Sulfide H2S Measuring Technologies
Summary
In Argentina, thousands of workers are exposed to hydrogen sulfide (H2S), a highly toxic and flammable gas. Industries such as oil and gas exploration, mining, petrochemicals, and water treatment rely on detectors to prevent personnel exposure. Selecting the appropriate detector is critical for safety, yet the selection criteria are often misunderstood. This document compares two sensor technologies—electrochemical and solid-state sensors—to aid in choosing the most suitable detector by outlining the advantages and disadvantages of each.
1. Motivation
No H2S detector available in the global market is perfect. Misunderstanding the type of sensor or the working environment can lead to catastrophic failures even in apparently functional devices. Key considerations include:
- Cross-Sensitivity: All H2S detectors exhibit some degree of cross-sensitivity, meaning they may respond to other gases in addition to H2S. In certain cases, the presence of another gas can inhibit the detector’s ability to sense H2S.
- Resource Optimization: The cost difference between detectors can be as high as 10-to-1. Proper interpretation of specifications, coupled with an understanding of the environment, can enable early warning systems at a fraction of the cost of alternatives. Conversely, false alarms can result in significant operational costs due to lost man-hours.
Additional factors to consider include measurement drift, repeatability, contamination resistance (poisoning), overload capacity, sampling systems, and filters.
2. Operating Principles: Solid-State Sensors
Solid-state sensors use a semiconductor material on a non-conductive substrate, with electrodes applied to it. The substrate is heated to facilitate changes in conductivity in the presence of H2S. In clean air, oxygen (O2) molecules immobilize electrons in the semiconductor, creating a high-impedance state. When H2S molecules displace O2 on the sensor’s surface, electrons are released, reducing electrical resistance. This change is logarithmically correlated with H2S concentration.
Weaknesses: Solid-state sensors exhibit cross-sensitivity to a broad range of gases, as mechanisms beyond O2 displacement affect impedance.
3. Operation Principles, electrochemical sensors
Electrochemical sensors function as combustion cells that convert chemical energy into electrical energy. These sensors contain two or three electrodes and an electrolyte separated by a membrane, which acts as a barrier to water. When gas molecules pass through the membrane, oxidation-reduction occurs, generating a measurable current proportional to the gas concentration.
Advantages: Electrochemical sensors are more selective than solid-state sensors. Manufacturers often provide cross-sensitivity tables, though field tests are recommended for gases not listed. In practice, these sensors are generally more reliable for detecting H2S.
Weaknesses: Certain gases can reverse oxidation-reduction processes, causing negative measurements.
4. Operating Principles: Optical Sensors
Optical sensors use light to detect H2S. These sensors rely on the absorption or scattering of light at specific wavelengths by H2S molecules. In typical designs, a light source emits a beam through the sample gas, and a detector measures changes in light intensity. The reduction in intensity corresponds to the concentration of H2S.
Advantages:
- High accuracy and fast response times.
- No cross-sensitivity to other gases in many designs.
- Suitable for remote sensing applications.
Weaknesses:
- High initial cost compared to electrochemical and solid-state sensors.
- Sensitive to dust, dirt, and other optical obstructions.
4. Calibration and Lifespan: Solid-State Sensors
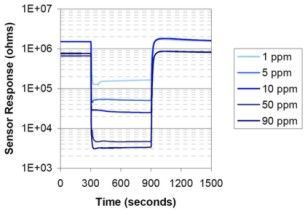
In clean air, the resistance of solid-state sensors is approximately 10⁶ Ω. This resistance decreases logarithmically with increasing H2S concentrations (e.g., 10⁵ Ω for 1 ppm and 10⁴ Ω for 100 ppm). Calibration requires at least two H2S concentrations to account for this logarithmic behavior. Long-term drift and a phenomenon known as “go-to-sleep” (insensitivity after prolonged exposure to clean air) necessitate periodic recalibration. Modern solid-state sensors mitigate these issues with improved designs. Their typical lifespan is 3–5 years.
5. Calibration and Lifespan: Electrochemical Sensors
Electrochemical cells typically last 6 months to 3 years, depending on resolution. Larger membrane surfaces increase sensitivity but accelerate electrolyte depletion. Calibration compensates for gain reduction, and zero drift is negligible. If calibration is missed, errors remain minor, often within acceptable thresholds for safety systems. Electrochemical sensors’ absolute zero and low drift make them ideal for low-concentration measurements.
7. Calibration and Lifespan: Optical Sensors
Optical sensors require minimal calibration since their measurements are based on stable optical principles. The typical lifespan of optical sensors is 5–10 years, depending on environmental factors and maintenance. Periodic cleaning of optical components is necessary to maintain accuracy.
6. Humidity and Temperature Effects: Electrochemical Sensors
Electrochemical sensors are unaffected by changes in humidity and operate reliably in 15–95% relative humidity. Temperature changes affect the H2S-to-current conversion ratio, but this is often compensated by built-in temperature controls, limiting errors to under 10%.
7. Humidity and Temperature Effects: Solid-State Sensors
Solid-state sensors are more robust in extreme environments, withstanding 0–100% relative humidity. However, direct contact with water can desensitize the sensor. Temperature effects depend on the presence of a temperature controller; uncontrolleEse articulo lo escribe yo, puede tener errores conceptuales involunbtarios, ademas tiene 15 años, ¿
con tu conocimiento puedes mejorarlo tecnicamente ya agregar una tercera tecnolgia, redactanto nuevamente el informe=d temperature variations can lead to significant errors, especially at low or high gas concentrations.
10. Humidity and Temperature Effects: Optical Sensors
Optical sensors are generally unaffected by humidity changes but may require dehumidifiers in extremely moist environments to prevent optical distortion. Temperature fluctuations can impact the light source and detectors but are often mitigated by temperature compensation mechanisms.
8. Maintenance and conclusions
Electrochemical sensors are generally more affordable, with replacement costs often below 50% of those for solid-state sensors. While solid-state sensors require more maintenance, they are better suited for extreme environments due to their durability. Optical sensors offer high precision and low cross-sensitivity but come with higher costs and require occasional cleaning of optical components.
Both sensor types—electrochemical and solid-state—require regular calibration, typically monthly or quarterly. Optical sensors, however, demand less frequent calibration, making them ideal for applications where maintenance needs to be minimized.
In conclusion, the choice of sensor depends on the specific application. Solid-state sensors are ideal for harsh environments, electrochemical sensors excel at detecting low concentrations with high precision, and optical sensors provide unparalleled accuracy and speed for critical applications.